Sinopharm Vaccine Wikipedia : China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says
This article provides a summary of those interim recommendations. This vaccine does not have a name.
Sinopharm Wibp Covid 19 Vaccine Wikipedia
Sinopharm vaccine side effects.
Sinopharm vaccine wikipedia. By May Sinopharm had supplied 200 million doses. Sinopharm vaccine side effects. Sinopharm expects to produce one billion doses of BBIBP-CorV in 2021.
Please participate on that page and not in this talk page section. The Sinopharm COVID-19 vaccine vero cell inactivated vaccine resource includes key information on the vaccine specific requirements. Tozinameran sold under the brand name Comirnaty is an mRNA-based COVID-19 vaccine developed by the German biotechnology company BioNTechIt is authorized for use in people aged twelve years and older in some jurisdictions and for people sixteen years and older in other jurisdictions to provide protection against COVID-19 caused by infection.
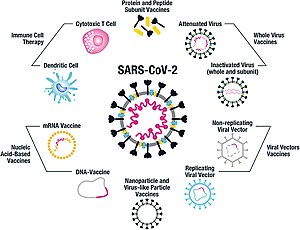
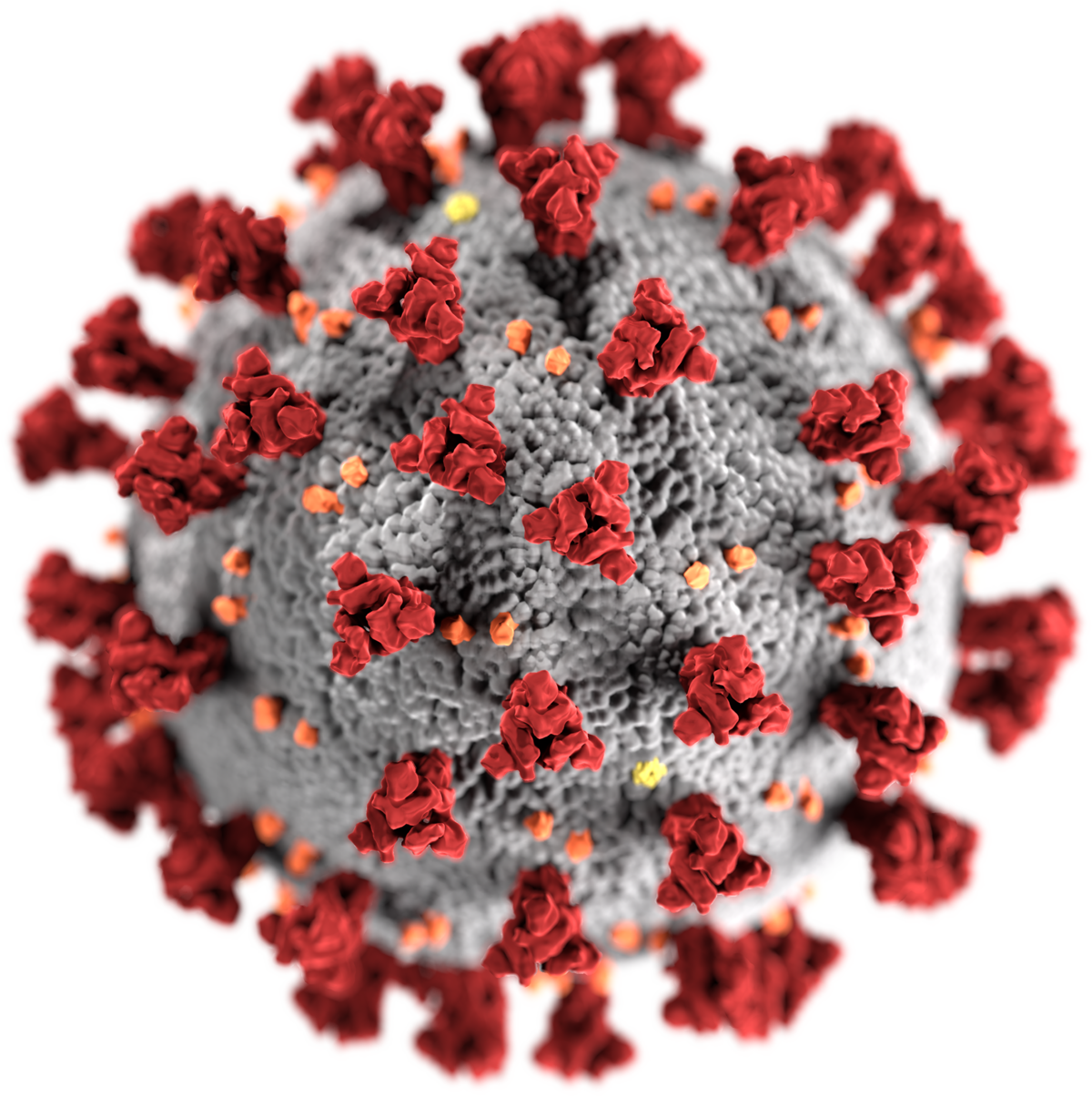
The company Sinopharm belongs to the government of China. A COVID19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 SARSCoV2 the virus that causes coronavirus disease 2019 Prior to the COVID19 pandemic an established body of knowledge existed about the structure and function of coronaviruses causing diseases like severe acute respiratory syndrome SARS and Middle. The Philippines has expressed its intent to get some of Canadas COVID-19 vaccines.
The other inactivated virus vaccine developed by Sinopharm is WIBP-CorV. The OxfordAstraZeneca COVID-19 vaccine sold under the brand names Vaxzevria and Covishield is a viral vector vaccine produced by the British University of Oxford British-Swedish company AstraZeneca and the Coalition for Epidemic Preparedness Innovations. Here is what you need to know.
On 7 May 2021 the World Health Organization approved the vaccine for use in COVAX. Sinopharm BBIBP-CorV COVID-19 vaccine instruction manual chnpdf. Move discussion in progress.
The Philippines has received about 1000000 Sinopharm COVID-19 vaccine donations on August 2021 from China. Denmark and Norway suspended the use of the OxfordAstraZeneca vaccine due to a small number of reports of a rare blood clot disorder. Bacillus Calmette-Guerin BRACE trial Edit.
The PfizerBioNTech COVID-19 vaccine INN. This article provides a summary of the interim recommendations. The other inactivated virus vaccine developed by Sinopharm is WIBP-CorV.
Posted by 5 minutes ago. The document has moved here. You may access the full guidance document here.
This document is the instruction manual of Sinopharm BBIBP-CorV COVID-19 vaccine which was written in. This vaccine started phase III trials in the middle of July 2020. The vaccine was given authorization by Chinas National Medical Products Administration on 31st December 2020 while it has been given authorization for its emergency use.
The result of the discussion was Moved to Sinopharm BIBP COVID-19 vaccine and Sinopharm WIBP COVID-19 vaccine. The WHO Strategic Advisory Group of Experts SAGE has issued interim recommendations for the use of the Sinopharm vaccine against COVID-19. The Sinopharm Vaccine or BBIBP-CorV has been developed by the Beijing Bio-Institute of Biological ProductsBBIBP in collaboration with the Sinopharm Vaccine or BBIBP-CorV.
EXPERIMENTAL COVID-19 VACCINES BIO-WEAPONS. The Philippines has received 100000 vaccines Sinopharm BBIBP-CorV vaccine from the United Arab Emirates. Hi im trying to find reliable source with sinopharm covid-19 vaccine side effects.
It is an inactivated virus vaccine. By May Sinopharm had supplied 200 million doses. There is a move discussion in progress on TalkBBIBP-CorV which affects this page.
Im surprised my usual source of information Wikipedia mentioned nothing about side effects. Sinopharm expects to produce one billion doses of BBIBP-CorV in 2021. Lina said on the Chinese social media platform Weibo that the COVID-19 vaccine developed by Sinopharm which is a Chinese state-run company is extremely unsafe for.
On 7 May 2021 the World Health Organization approved the vaccine for use in COVAX.

Sinopharm Bibp Covid 19 Vaccine Wikipedia
Chinese State Backed Firm Expects Coronavirus Vaccine Approval For Public Use Within Months Reuters

The Cold War Diplomacy Behind Covid 19 Vaccines

Chinese Vaccine Candidate Effective Against All Known Coronavirus Strains Sinopharm Global Times
/cloudfront-us-east-2.images.arcpublishing.com/reuters/K7IMX7V5NZOOVGSOPKVPNUA6MY.jpg)
Sinopharm S Two Covid 19 Shots Effective Study Says Reuters

Vaccine Diplomacy China S Global Vaccine Efforts And Controversies China Institute

Oxford Astrazeneca Covid 19 Vaccine Wikipedia

Sinopharm Bibp Covid 19 Vaccine Wikipedia
China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says
Sinopharm S Two Covid 19 Shots Effective Study Says Reuters

Pfizer Biontech Covid 19 Vaccine Wikipedia
Covid 19 Vaccine Development Clinical Trials Gvn
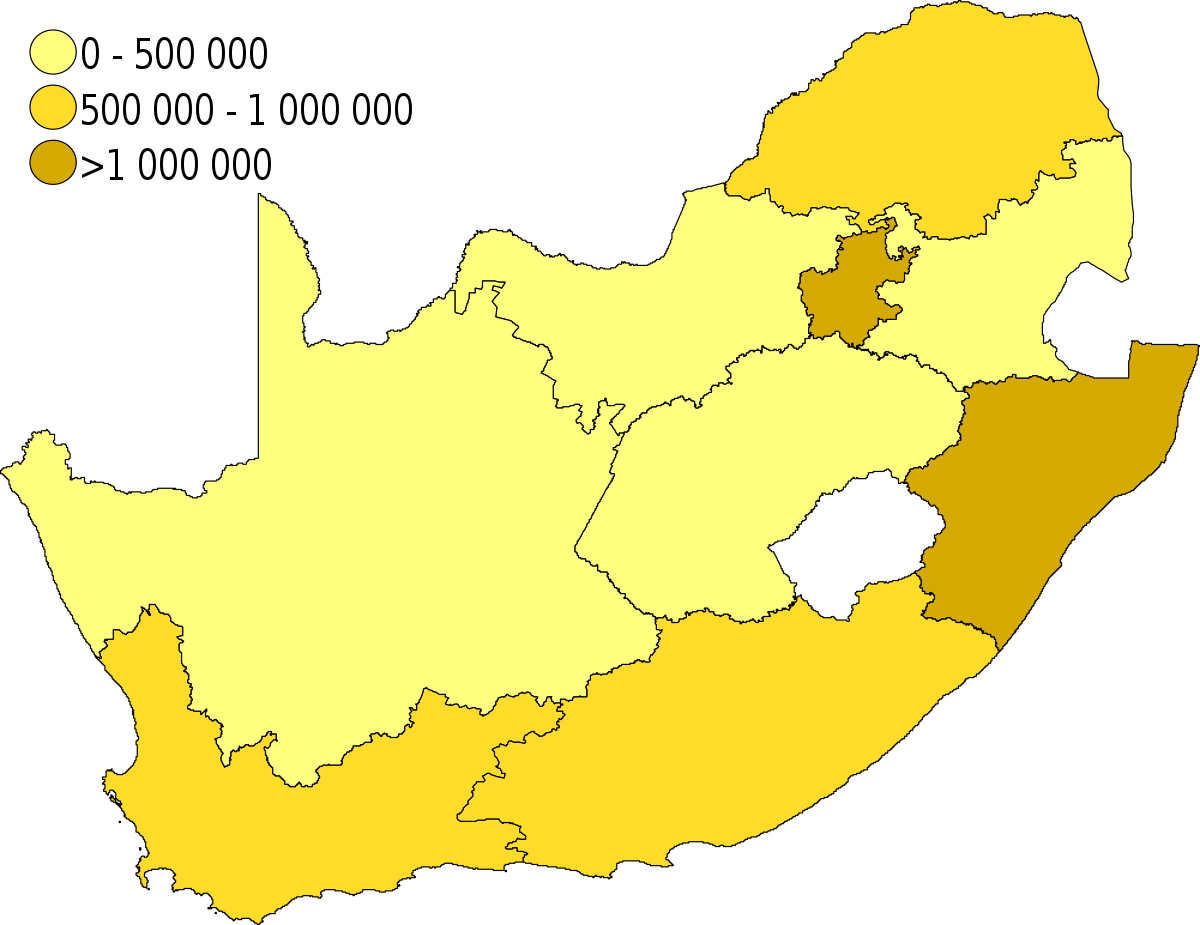
Covid 19 Vaccination In South Africa Wikipedia

Uae Says Sinopharm Vaccine Has 86pc Efficacy Against Covid 19 World Dawn Com